CMMC Ongoing Compliance Management
Maintain your CMMC certification with ongoing monitoring, evidence management, and regulatory updates—stay audit-ready between assessments.
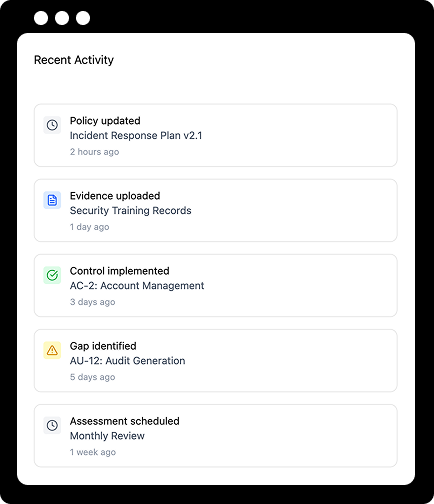
Validate Readiness
The degradation problem
Certification isn't forever

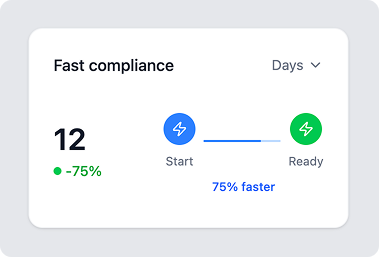

Set it and sustain it
Sustainable Compliance

Proactive, Not Reactive
Our Aproach
Quarterly Reviews
Change Management
Evidence Management
Training & Support
Why ongoing management?
Protect Your Compliance Investment

Pass re-assessment as easily as initial certification
Fix problems before they fail audits
We handle monitoring so you focus on delivery

Scale confidently knowing compliance keeps pace
Stay certified while competitors lapse
Comprehensive support
Everything You Need To Stay Compliant
Ongoing Compliance Support

Quarterly Compliance Reviews: Systematic testing of all controls with findings report

Evidence Management: Continuous collection, organization, and validation

Change Impact Analysis: Evaluation of new systems, tools, or processes

Regulatory Monitoring: Alerts on CMMC updates with impact assessment

On-Demand Support: Unlimited guidance calls for compliance questions

Annual Mock Assessment: Validation review to confirm continued readiness

Triennial Re-Assessment Prep: Full preparation for your next C3PAO evaluation
Faq
We’ve got you covered, 24/7.
Frequently asked questions.
Immediately after certification or anytime post-assessment
Yes, flexible terms allow pausing during slow periods
No, we guide your team, keep you updated in terms of compliance changes and validate compliance
By staying compliant there is minimal work in preparing for the re-assessment.





.svg)
.svg)

